Cervical Cancer Screening Strategies
Understanding Cervical Cancer in the United States
The pap test is a significant accomplishment in cancer screening history, cutting cervical cancer deaths by more than 70% since its introduction 80 years ago.1,2 Unfortunately, incidence of cervical cancer is no longer declining, making it essential that healthcare professionals continue to utilize the most comprehensive screening approaches available.2
4,320
estimated deaths in 20253
13,360
estimated new
cases in 20253
7.7
incidence rate in
2017–20213*
USPSTF 2018 Guideline Recommendation4
For women ages 21–29 years:
Screening with cervical cytology alone every 3 years is recommended.
For women ages 30–65 years old:
For women ages 65 years and older:
Do not require screening after adequate prior negative screening results.
Cervical Cancer Screening Collection Methods and Results

Clinician-collected (Women aged 21–29)
(co-testing)
Clinician-collected
Clinician-collected

Patient-collected
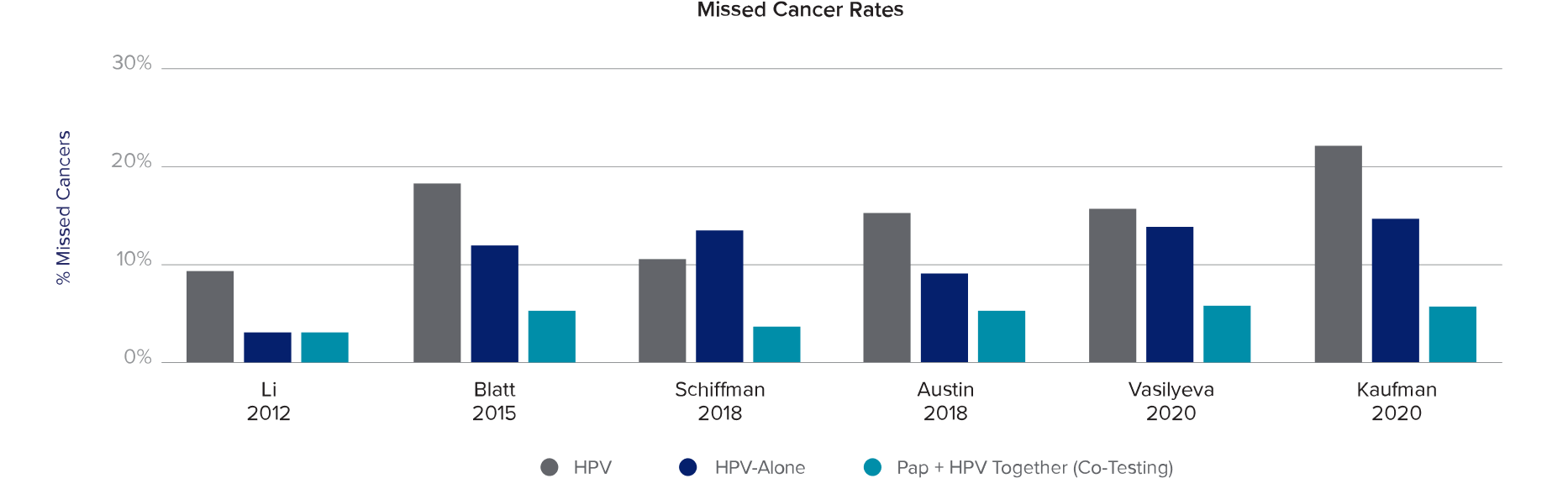
Pap + HPV (Co-Testing) Missed the Fewest Cancers and Pre-Cancers5
Retrospective longitudinal study of cervical cancer screening involving:
- Women 30–65 years of age
- 1,615 co-tests preceding 1,259 cases of cervical cancer
- 11,164 co-tests preceding 8,048 precancer diagnoses (CIN3/AIS)
Head-to-head performance matched to longitudinal biopsy
- Pap- Alone
- HPV- Alone†
- Pap + HPV (co-testing)
Key Findings from Multiple Screening Studies
1 in 5
cervical cancers were missed with HPV- Alone‡7,8
70%
of the cancers missed by HPV- Alone were identified by Pap + HPV (Co-testing)‡5
95%
of cervical cancers were detected with Pap + HPV (Co-testing)7,8
What About HPV Self-Collect?
A self-collect vaginal swab is collected by the patient in a healthcare or private setting and for individuals not currently participating or engaging in routine screening.16-18 Clinician-collected cervical samples are preferred by American Society for Colposcopy and Cervical Pathology (ASCCP).19
Risk of Missed Disease
“… the primary risk associated with self-collected vaginal specimens may arise if regularly-screened individuals electively switch from clinician-collected cervical specimens to self-collected vaginal specimens, which could result in potential missed cervical cancer disease cases that could have otherwise been detected and prevented using the current standard of care (i.e., clinical-collected cervical specimens).” —FDA17,18
Clinician-Collected Cervical Samples Remain the Standard of Care
“…self-collect vaginal specimens appear less sensitive and specific in comparison to clinician-collected cervical specimens.” —FDA regarding HPV self-collect17,18
HPV Self-Collection Comes with Trade-Offs19
Loss to Follow-Up
There is nearly 4x more loss to patient follow-up compared to clinician collection.
Triage Testing
Additional testing not possible with vaginal specimen. Positive results require clinician cervical exam or speculum specimen collection.
Repeat Testing
Self-collected tests are recommended every 3 years following negative results.
Cytology is Needed in the Following Scenarios:19
- HPV 16/18 positive: requires immediate diagnostic evaluation (e.g. colposcopy) and ongoing surveillance§
- HPV-positive, genotype unknown
- HPV HR12 (other) positive
- HPV 45, 33/58, 31, 52, 35/39/68, 51 positive
- Symptomatic patients
- Immunocompromised patients
- During surveillance
HPV Self-Collection: A Step Forward or a Step Back?
The Netherlands HPV Self-collect Case Study
%
decline in screening participation from 2017–202220-22
%
of patients did not follow up after receiving a positive hrHPV test result23
%
decreased sensitivity vs. clinician sampling in an adjusted population23
Most patients still prefer to visit their GP20
Self-sampling is increasing, however, most self samplers are “switchers” who previously received a pap test, rather than new, untested patients23
Support Patient Preference With Clinician-Collected Co-Testing24
Co-testing was a preferred screening method among adequately and under-screened women
HPV self-collect was the least preferred method of screening among adequately screening women
Under-screened women prefer co-testing over HPV self-collect
All women regardless of screening modality prefer to start screening at the age of 21
The Affordable Care Act Covers Many Co-Testing Cases
For patients this may mean:25
No co-pay
No deductible
No out-of-pocket cost
Note: Patients should consult their healthcare plans to verify coverage
Hologic’s Offerings
Our suite of testing tools leads the market in accuracy and sensitivity, providing the insights you need for your patient’s best care.
Aptima® HPV 16 18/45 Assays
Aptima® HPV 16 18/45 Assays
Detects high-risk HPV types with high sensitivity and specificity to identify women at risk for cervical disease.
ThinPrep® Pap test
ThinPrep® Pap test
Liquid-based cytology for early detection of cervical cytologic abnormalities, including precancer and cancer.
Let’s Connect
Have a question or need to talk to a Hologic team member? We’re here to help.